“Dead Cthulu waits dreaming…” H.P. Lovecraft
In the 1930s, pulp horror writer H.P. Lovecraft penned tales of ancient monsters called Old Ones that, if awakened, would emerge to devour the world. One of these horrors, Cthulu, lay in death’s sleep in his house called R’lyeh at the bottom of the Baltic Sea (Charles Stross) awaiting some impetus to disturb him from necrotic slumber (ironically, the Baltic sea bed contains one of the world’s highest concentrations of the deadly hydrogen-sulfide producing bacteria that are a focus of this article).
(2007 Hydrogen Sulfide emission off the coast of Namibia. Such emissions tend to color the surface water green and, in extreme cases, black. Image source: Earth Observatory)
In the imaginary world of H.P. Lovecraft, terrible lore of these horrific Old Ones, among which, Cthulu was the worst, lay stored in ancient tomes. To learn of these mysteries was to risk madness. For the Old Ones were too awful for the human mind to conceive without succumbing to a hopeless darkness.
In researching the terrors that could emerge in a world destabilized by human warming, I am often reminded that human imagination is not without a sense of dramatic irony. But in this case, the irony invoked is that human imagining, in fiction, seems to sometimes possess a broader perception of potential real world risks and their implications for human thought, than the far more defined warning signal coming from the sciences.
Cthulu, in this case, may as well be a metaphor for one of the worst of the world’s ancient climate horrors — the oceanic production of hydrogen sulfide gas that occurred from time to time, during various hothouse events. A production implicated in many of the worst mass extinction events ever to mar the history of life on Earth.
Hydrogen Sulfide — Bi-product of Bacterial Metabolism in the Ancient Oceans
In understanding this ancient horror, we must first take a look at some of the world’s oldest and smallest creatures. Primordial bacteria.
About 3.5 billion years ago, the Earth was a hot, toxic place, bombarded by solar radiation. It was still cooling down after its initial formation. The oceans had spilled out over its surface, but the continents had yet to emerge. Atmospheric levels of CO2 were high and oxygen was virtually nonexistent.
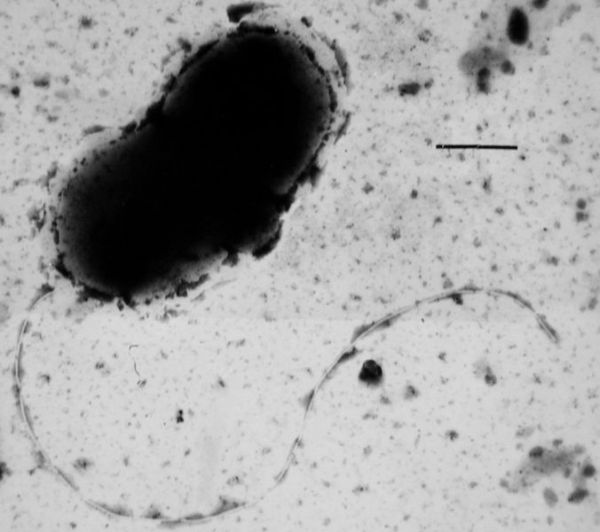
(Desulfovibrio vulgaris, one of the most well-researched hydrogen sulfide producing bacteria. Image source: Commons)
But, in this world, small microbial organisms thrived. Deprived of oxygen, which is the now typical means of respiration for non plant organisms, the microbes required other sources for their simple cellular metabolism. Sulphate was common in the world’s emerging oceans and reacted well with hydrogen, which was also very common. The result was the emergence of some of the oldest known living organisms — the sulphate reducing bacteria.
Suphate reducing bacteria combined sulphate and hydrogen to produce hydrogen sulfide gas or H2S.
As a result, ancient oceans were cauldrons bubbling over with hydrogen sulfide which was the biproduct of these primordial organisms’ respiration in much the same way that oxygen is a biproduct of plant respiration and CO2 is a biproduct of animal respiration. Such an ocean state, called a Canfield Ocean by today’s scientists, was the common state for the world’s oceans until the emergence of more complex life around 2.5 billion years ago. By about 600 million years ago, the Canfield Ocean state only very rarely came into being and when it did, mass death tended to rapidly follow.
Changes Came With the Emergence of Oxygen
As the Earth system matured and new organisms came into being, CO2 reducing photosynthetic life emerged and began to produce an abundance of oxygen. Toxic to the ancient organisms, the abundance of oxygen pushed the sulphate reducing bacteria into the world’s low-oxygen corners. The deep ocean, or anaerobic mud became a haven for these tiny primordial monsters. Never again would they dominate as they once did. But, from time to time, when priomordial ocean states would infrequently emerge during various hot-house phases in Earth’s climate progression, these life forms would explode, producing prodigious volumes of what, to more complex life, was the very toxic hydrogen sulfide gas.
A Toxic, Volatile Gas
Hydrogen sulfide is directly toxic to most plant and animal based life. Its effects in animals are similar to that of hydrogen cyanide in that it eventually results in cardio-pulminary shock and then death. Lower levels of hydrogen sulfide are associated with loss of smell, blindness, respiratory infections, and loss of neurological and nervous system function. At very low levels, hydrogen sulfide is non toxic and is even produced in cells to perform various functions. Human lethality begins at around 600 parts per million. Smaller mammals with higher respiration rates begin to show lethality at around 450 ppm. Doses in the range of 10-20 parts per million have been known to cause eye irritation and damage over long periods of exposure. Levels over 50 ppm are generally considered harmful if exposure occurs for long durations. Doses between the irritation dose (10 ppm) and the lethality dose (600 ppm) over extended periods are shown to cause the eye damage and degenerative nerve and lung changes listed above.
In the environment, hydrogen sulfide causes numerous other damaging impacts. The gas reacts with hydroxyl and oxygen over the course of about 1 to 3 days to produce sulfur dioxide. Aside from providing a mechanism to draw down local oxygen levels, the sulfur dioxide product can end in the stratosphere where it substantially degrades the protective ozone layer.
Though hydrogen sulfide is slightly heavier than air, tending to pool at lower elevations, it is light enough to be born aloft by winds to various layers of the atmosphere and its even lighter sulfur dioxide products are quite a bit more mobile. At high enough atmospheric concentrations, both it and its sulfur products could begin to seriously degrade the Earth’s protective ozone layer. And evidence exists in the geological record of such events occurring on at least a couple of occasions during the last 250 million years. Notably, during the Permian extinction event, large numbers of fossils have been found with the characteristic UV damage that would occur in a world in which the ozone layer had been greatly degraded.
At high enough concentrations, hydrogen sulfide is volatile enough to burn. A 4.3 percent concentration is immediately combustible, producing a bluish flame. This extraordinarily high concentration would be almost immediately lethal to humans if inhaled and usually only presents a fire risk at highly concentrated sources.
In the current day, high concentrations of hydrogen sulfide gas are often associated with natural gas extraction. Natural gas, by volume, can contain as much as 90 percent hydrogen sulfide. The hydrogen sulfide, in this case, occurs due to catalytic reaction of the hydrocarbon with certain minerals present in the Earth. Though not produced by the same mechanisms as oceanic hydrogen sulfide, the gas in this form is just as dangerous and is a constant concern to workers of the oil and gas industry. Notably, risks of hydrogen sulfide exposure, leaks, and release into the environment have greatly increased with the widespread adoption of hydro-fracking practices that use high pressure liquids to rupture tight gas deposits and chaotically release the substance for its collection at one of the US’s 1 million well sites.
In general, the volatility, danger, and toxicity of the gas is difficult to overestimate. Notably, its lethality resulted in its use as a chemical weapon during World War I.
Culprit of Past Mass Extinctions
High concentrations of hydrogen sulfide, resulting both from its production in a Canfield type ocean state and, possibly, through its release in large methane pulses from the sea bed during catastrophic warming events, has been implicated in numerous mass extinction events both on land and in the ocean. Notably, the Permian-Triassic extinction, the Triassic-Jurassic extinction, and the PETM extinction in the deep oceans all show signs related to ocean anoxia and varying levels of hydrogen sulfide gas production. Earlier mass extinctions such as the Devonian and Ordovician extinctions were also likely caused by anoxia and related hydrogen sulfide production. Lesser extinctions in which ocean anoxia also probably played a part include the Ireviken, Mulde, Lau, Toarcian and Cenomanian-Turonian events.
Prominent researchers such as Ward and Kump propose that hydrogen sulfide production by sulfate reducing bacteria is a primary extinction mechanism in stratified and anoxic oceans due to their inevitable multiplication in these environments which are, to them, far more favorable than oxygen-rich mixed oceans. In a Canfield Ocean world, large, episodic releases of hydrogen sulfide gas would cause local mass poisonings of land dwelling animals, especially of those living near large ocean-linked bodies of water. The ocean itself would be brimming full and spilling over with this nasty substance. This condition would be highly toxic to most life, requiring extreme adaptation to survive in naturally occurring havens.
Separate depletion of atmospheric oxygen through both the plant killing mechanism of hydrogen sulfide gas and its long-term reaction with oxygen would also make life far more difficult to terrestrial creatures. Finally, the massive amounts of sulfur dioxide produced in such a world would combine with the hydrogen sulfide pulsing into the atmosphere to create an ongoing, long-term degradation of the ozone layer, further harming surface dwelling plants and animals.
During the Permian Extinction, such conditions, together with other impacts of a global hothouse featuring a massive flood basalt, are thought to have wiped out more than 70% of terrestrial organisms and a total of more than 95% of all life on Earth.
Occurrence in Current Seas
(Expanding bottom anoxia, hypoxia and hydrogen sulfide production since 1960 in the bottom zone of the Baltic Sea. Red indicates region experiencing low or no oxygen content. Black indicates areas where H2S gas is detected. Image source: Baltic Sea Trends)
The world’s oceans, according to recent research, are rapidly becoming more stratified and less oxygen-rich. The result is that mixing between various layers of the ocean is beginning to shut down reducing oxygen content in the deep ocean and spurring the expansion of numerous oceanic dead zones.
Over the past 150 years, the Pacific Ocean was observed to become more stratified at a pace ten times that seen during the end of the last ice age about 12,000 years ago. Such a rapid pace of stratification is putting severe stress on the world’s oceans with numerous regions showing the effects of low oxygen (hypoxia) and some regions succumbing to increasingly anoxic states.
These low oxygen events have been associated with multiplying oceanic dead zones. Very large dead zones have been observed in the Pacific, specifically off the coast of Oregon. Other major dead zones continue to be observed at the mouth of major river systems, such as within the Gulf of Mexico, where the appearance of massive related toxic algae blooms is now an almost annual event. In general, almost all ocean dead zones are expanding leading to the dramatic reduction in habitat size of numerous fish species. And even the most cursory research provides ample evidence that ocean hypoxia is expanding concurrently with a rapidly expanding ocean stratification.
When combined with the jarring effects of rapid ocean warming and expanding acidification, it becomes plainly obvious to almost any ocean ecologist that the world’s ocean system is suffering the heavy bombardment of a new mass extinction event.
It is this kind of low or no oxygen environment that is a prime breeding ground for hydrogen sulfide producing bacteria. In numerous places around the world, such as off the coast of Namibia, in the Black Sea, in the Baltic Sea, in the Gulf of Mexico, in the Chesapeake Bay, and off the coast of Oregon, large and expanding zones of hydrogen sulfide have been observed in deep water environments. In some regions, this hydrogen sulfide occasionally penetrates to the surface layer resulting in major fish kills and a concordant rotten egg smell.
Off the Oregon coast, in perhaps one of the most extreme examples of ongoing ocean hypoxia, one of the world’s largest and most oxygen-starved dead zones continues to expand. The oxygen levels in this region are so low that local fisherman often bring back horrific tales of baby bottom dwelling creatures such as crabs and octopus climbing anchor ropes to escape the dangers of their oxygen-starved environment. In another, possibly related event, masses of starfish perished during 2013 and 2014 as they, over the course of a few weeks, turned to goo. The fact that this sci-fi esque mass death of starfish occurred near one of the world’s largest dead zones should not be lost on those concerned for world ocean health.
But perhaps even more concerning is the fact that this region off the Oregon coast is producing substantial volumes of hydrogen sulfide gas. Volumes high enough in concentration to occasionally cross the ocean-air boundary.
Oregon possesses numerous features that would aid in the transport of this gas to the surface. Primarily, the near Oregon ocean system frequently features strong up-welling currents. These currents can push bottom waters through stratified layers and cause them to contact the surface. If these oxygen starved bottom waters contain hydrogen sulfide gas, as they increasingly do, this harmful gas can be transported into the local atmosphere through mixing.
Such events, thus far, have been limited. However, since the Oregon dead zone’s discovery in 2001, its expansion has been both deeply concerning and well documented, showing a rapid and dangerous growth over the 13 years since its emergence. Despite the documented expansion of deep water hydrogen sulfide in numerous oceanic regions, the only other ocean zone on Earth observed to emit hydrogen sulfide gas to the atmosphere is in the region of coastal Namibia.
In Namibia, huge volumes of organic compounds fall into the sea after being flushed down ocean terminating streams and rivers. These organic compounds rain down into the deep ocean directly off Nambia’s coasts. There, the ocean bottom hosts both an anoxic environment and masses of hydrogen sulfide producing bacteria. As a result, toxic hydrogen sulfide gas periodically erupts from the ocean and into the atmosphere there.
The Very Real Threat That is Oceanic Hydrogen Sulfide Gas Production
There are few limiters to oceanic hydrogen sulfide production in the world’s increasingly stratified and oxygen starved oceans. Sulphate, which the bacteria require for respiration, is one of the most common ocean elements. In the current ocean, it is present in volumes greater than those seen during the Permian Extinction when these tiny monsters are thought to have done their worst.
Iron and manganese in the world ocean system aids in the development of less permeable boundary layers that help keep a lid on deep ocean concentrations of hydrogen sulfide. However, even in the anemic circulation of stratified and Canfield oceans, upwelling will bring the gas to the surface in certain regions. In addition, as the oceans contain greater and greater volumes of the toxic gas, it will push closer and closer to the surface, rendering metals that help reinforce the boundary layer a practically useless prophylactic (such high metal concentrations currently prevent hydrogen sulfide from penetrating the surface layer in the Black and Baltic Seas as well as in the Chesapeake Bay).
In addition, modern industrial farming practices provide extra nutrients upon which these dangerous microbes can feed. High levels of hydrogen sulfide in the deeper regions of the Chesapeake Bay, for example, owes its existence, in part, to massive farm run-off into the Bay and the dumping of mass volumes of nutrients upon which the sulphate reducing bacteria can feed.
It is important to note that we observe heightened levels of hydrogen sulfide gas in the world ocean system now. As hypoxia and anoxia progress with the human-caused warming of the oceans, and as glacial melt interrupts and alters the now strong ocean currents and related mixing, it is certain that hydrogen sulfide production in the deep ocean will continue to increase resulting in elevating levels of harm to ocean dwelling animals and ever more numerous instances of hydrogen sulfide gas contact with coastal and surface waters.
Dead Cthulu Rises
In the context of increasing ocean hypoxia and stratification, we might do well to remember that we are tiny, weak beings at the mercy of great natural forces which we can barely conceive or understand. Forces that we have unwittingly, callously and ignorantly set into motion.
* * * * *
Long ago, when I was a ten year old child, I was fortunate enough to meet an amazingly kind, adventurous and inquisitive man. The man, whom I will call Rick to keep safe his identity, was a bit of a local paramour in ocean and bay research. He was constantly in contact with both the ocean and adjacent Chesapeake bays, ever venturing out to explore and to conduct research on marine life. In later years, he would be the impetus behind annual summer marine science camps hosted by the Virginia Institutes of Marine Science, Norfolk Academy, and Old Dominion University. But this was later. Now, Rick was helping an elementary school student present on the issue of our then expanding understanding of marine science.
Living so close to the bay and ocean, I was intimately in contact with the living boundary of land and sea. In the more demanding and less stimulating forum that was public education, I seldom had the opportunity to indulge my passion for the oceans. But at age 10 I was given the opportunity to give a broad marine science presentation for my classmates. As part of my project, I constructed posters and models depicting the current state of world ocean research. I graphically illustrated the various known zones of the bathysphere, the light and life filled ones and the more mysterious and far less well understood depths. But Rick was the centerpiece of my presentation. He was my keynote. And he energetically answered all my own and fellow students’ questions, speaking in the kind and intriguing manner that would later draw so many into his charismatic orbit.
In later years, I would attend Rick’s summer marine science camps on two different occasions. In both cases, I observed what appeared to be an increasing concern about both the health of the Chesapeake Bay and the neighboring oceans. In later years, Rick’s attitude, once so full of optimism, bordered on cynicism. The world he loved so deeply was experiencing death on a scale that horrified him. And he harbored a deep sense of betrayal that we weren’t doing more to stop the senseless slaughter of so many of the living things he saw as both beautiful and wondrous.
In the mid 2000s, Rick committed suicide. To me, one of the great ocean pioneers of my developmental years had passed away by taking his own life. And I couldn’t help but wonder if the horrible ways in which the oceans that he so loved were changing was just too much for him. If the commercialization and cheapening of all the things he held most dear along with their subsequent damaging and putting at great risk of terrible harm had robbed his life of beauty and purpose.
Rick was, if anything, a very intelligent and sensitive man. He knew what was happening to the Bay and ocean on a personal level. When the Bay was harmed it was as if it hurt Rick too.
Rick also knew how temperature changes affected the depths. For he was on the front line studying it. He was hauling up the fish and the water samples. He was doing the measuring with his own hands.
Was the awakening of terrible Cthulu, in the form of hypoxia, anoxia and deadly hydrogen sulfide producing bacteria, too much for Rick to continue bearing mute witness? Did his pleas to those working in the marine science community fall only on deaf ears? Was it just too much for this sensitive, feeling, and intelligent man to bear?
* * * * *
If Rick taught me anything it was that our lives and the life of the ocean are deeply connected. One cannot remain healthy without the other. In contrast to this basic understanding, the damage our continued industrial emission of greenhouse gasses is doing to the world ocean system is a horrific travesty. And the damage we have already caused, have already done to those most sensitive creatures among us, have already set in play for future decades and centuries, is tremendous.
The ocean suffocates, bleeding deadly hydrogen sulfide gas. Cthulu rises from his ancient house in the depths. And yet we still continue down the wretched path in pursuit of more terrible things to come.
Links:
Through the Looking Glass of the Great Dying
Toxicological Profile for Hydrogen Sulfide
Positive Reinforcement, H2S and the Permo-Triassic Extinction
Expanding Ocean Dead Zones are Shrinking Marlin, Tuna, and Billfish Habitats
Dead Zone Causing Wave of Death off Oregon Coast
Information about Hydrogen Sulfide in the Baltic Sea
Residence time for Hydrogen Sulfide in the Atmosphere








Miep
/ January 21, 2014Reblogged this on There Are So Many Things Wrong With This and commented:
Heartbreakingly good post, Robert.
LikeLike
antilandscaper
/ January 21, 2014Thank you Robert, for another thought provoking post for I find all your posts most interesting.
For myself, I always considered the endless changes to our ecosystems to be what finally bring us and so many species down, knowing that climate change as a contributing factor, but after reading your and other posts I feel it is as important as all the other harms we are doing in the name human superiority. So thank you for your hard work putting these together
Also I would like I your permission to re-blog this post and time to time others
LikeLike
robertscribbler
/ January 21, 2014Thank you for the kind comment. And I agree. I understand, I think, now why ecologists are so pessimistic.
Warmest regards and please feel free to reblog with impunity.
LikeLike
susan2196
/ January 21, 2014Reblogged this on abraveheart1.
LikeLike
Patricia
/ January 22, 2014Thank you for another enlightening and moving essay. You are one reason that I am so grateful to have access to the internet.
LikeLike
Tom
/ January 22, 2014Robert – now that’s how you investigate something you only had a little knowledge about. Spectacular job!
LikeLike
robertscribbler
/ January 22, 2014Happy to please Tom. Quite a bit of work and sleuthing for this subject. Still ongoing.
LikeLike
publiusmaximus
/ January 23, 2014Great post. Frighteningly portentous.
What about negative feedback loops that remove methane?
Also, since we know that the earth system has had CO2 levels this high in the past, and higher temps, but without experiencing a mass die-off and mass release of hydrogen sulfide, what might be the variable that allows or prevents it?
LikeLike
robertscribbler
/ January 23, 2014It’s not just how levels compare to the past, it’s the current pace of change and the size of the initial forcing.
IF all levels of CO2 were to remain at 400 ppm and all other GHG were to stabilize, then we’d only experience some increased ocean stratification, about 10-80 feet of sea level rise over the centuries, and wrenching changes to our weather.
The H2S release scenario comes into play moreso under a continued BAU emission and a more sensitive Earth Systems response. Unfortunately, with the risk of large methane release in the cards, we need to take severe ocean warming and anoxia into consideration as a risk even if human GHG emissions flat-line.
Negative feedbacks to methane release include absorption in the water column, methane consuming bacteria, and the relatively short residence time of methane in the atmosphere. The problem is that, with volumes of methane so large, even a small fractional release can quickly overwhelm any natural feedback mechanism and kick warming into a much higher gear.
It is worth noting that we already have a very substantial methane emission coming from the Arctic. It is not yet the size of a major release. But it does appear to be growing. And that should be serious cause for concern.
LikeLike
ccgwebmaster
/ January 24, 2014Interesting post – I learned some things. I’m curious to check my understanding – I don’t rate hydrogen sulphide very highly as a direct mass kill mechanism due to the localised nature of releases from the ocean and short residency time in the atmosphere. I grant that the wrong wind could kill large numbers of people if it blew onto a heavily populated area though.
The note about destruction of ozone is interesting and had slipped my mind. For anyone who wants a lot of bedtime reading, I suggest http://www.killerinourmidst.com/ as being worth a look (if memory serves). It deals with the end Permian mass extinction and explores (in considerable depth) the implications of a methane catastrophe including the release of hydrogen sulphide and destruction of the ozone layer.
It is notable not just for the amount of detail and length of the text there but also for being relatively old – it seems to have been written before the discovery of even the smaller methane plumes in the ESS (East Siberian Shelf) in 2008 – and indeed I found it no later than that myself (hunting as I was for methane information back then). To my mind we are firmly on track for a big methane problem if not a true methane catastrophe.
Anyway that’s an aside – I’m mostly curious if you think hydrogen sulphide is really an extinction level threat directly, or if you would agree with my view that it’s mostly a problem (at a global scale) due to the destruction of ozone? (which would greatly complicate life for those surviving on such a planet)
In terms of local threat potential I might note that tectonic activity including volcanoes can reasonably be expected to significantly increase as a result of climate change – that is also a potentially non trivial localised hazard. How would you compare the two sources of threat, were you contemplating a life in the future world we face?
LikeLike
robertscribbler
/ January 24, 2014I want to say that, in no small part, your comments on the issue of hydrogen sulfide here and over at the Arctic Ice Blog caused me to dig more deeply into the issue of hydrogen sulfide gas. So I owe you some thanks for spurring me to enquirer further.
When we look at most mass extinctions, it’s compounding sudden and ongoing impacts that tend to cause the damage. At the KT, we add a massive initial explosion, week long fires over much of the globe, and then a multiple years long severe climate change and reduction in sunlight reaching the Earth. Rapid die off, rapid recovery. What one would expect from a large impact.
The climate change die offs are more messy. We usually have a large flood basalt and all the troubles related to it. And we usually have a stratified or Canfield Ocean. We have evidence of major methane releases. And we typically have hothouse states.
This is what we see in the geology. But we have to engage imagination to envision what this means ‘on the ground’ or ‘in the sea.’
One thing I found in my investigation is that the is quite a lot of hydrogen sulfide in the oceans. Huge pools of it anywhere there’s a hypoxia or anoxic bottom. In the more stagnant seas, like the Black Sea, or in areas affected by our runoff, the related dead zones are essentially great pools of the stuff. The Chesapeake Bay and the Gulf of Mexico both feature deep water regions of hydrogen sulfide, for example.
Now a border, called a chemocline, tends to keep a cap on this nasty stuff, keeping it well below the surface. Occasionally, during periods of excessive warmth and hypoxia, or during times when nutrients cause oxygen depleting and anaerobic bacteria to explode, the chemo line can swell upward, invading the life filled zones and causing massive fish kills.
This is the state of regions of current seas and oceans. And I doubt, when compared with the oceans of even 30 years ago that this is either healthy or normal.
In a runaway hothouse, you end up with oceans in which the chemocline is close to the surface or contacts the over very large regions of ocean. These areas would emit h2s almost constantly. In addition, fluctuations in ocean states and temperatures would move the chemocline about causing spontaneous eruptions and out gassing. And in a hothouse world almost any major body of water would also suffer the same effects. So imagine a world in which approaching the water or residing near the water results in potentially lethal consequences.
So we already have a very hazardous world, and that’s just from the effects of h2s locally.
Now let’s thing about that methane release you mention. The methane comes from the sea bottom, exactly in the zones where h2s is densest. And it’s release provides a mechanism by which the h2s can be transported to the surface and released into the atmosphere. So imagine, during these huge methane pulse events, the concordant massive and sudden delivery of immense volumes of h2s to the atmosphere and up through the water column.
This would, potentially have a major lethality impact and may be one reason why the Permian extinction pulsed three times or thereabouts.
More later…
LikeLike
ccgwebmaster
/ January 25, 2014There are a several interesting (and potentially important) areas that I think need explored with hydrogen sulphide. It’s an interesting point you make about the scope for release concurrently with methane clathrates – although I think it would take some evidence or explanation to demonstrate feasibility of such mechanisms (eg how long does it take to produce the sulphide in deeper cooler water given slow bacterial growth and need for methane to feed it). However in principle one could (if able to postulate a physical mechanism and chronology to such an event) take the largest submarine craters we know about (~11km) and attempt to derive some imagination of the magnitude and implications of such an abrupt and localised release (how much gas and how big a kill zone under given wind conditions). In fairness the methane alone might pose a local threat from scope for explosion or asphyxiation in such large releases.
Likewise it would be interesting to have data to explore to what extent chronic non abrupt releases could pose issues to people existing in such a world – what areas would be particularly dangerous and what strategies could mitigate the danger.
I do not subscribe to (and feel the need to resist) the Guy McPherson assertion that humanity is necessarily committed to near future extinction, but there are nonetheless plenty of valid questions (including to do with hydrogen sulphide) that are inadequately explored. I do think it worth noting that even in the end Permian extinction some lifeforms (including some complex vertebrates) apparently made it through this period. Even in the worst case outcomes it is hard to see that extinction is an inevitable outcome for our species (although it is possible).
I would wager many people (even those who think they are well informed about climate change) aren’t even aware of the link with hydrogen sulphide (and it’s extremely high toxicity) and hence anything to bring their attention to it is arguably valuable (and while I might argue against it as a guarantor of human extinction, I can hardly say it’s a good or even negligible matter).
I wonder sometimes – people have this concept that bad things will happen, but how many think it is just a matter of inconvenience, of worse weather, rising food prices, floods and droughts – versus what we are on track for – foreseeable death of billions from hunger and violence and ultimately potential transformation of the planet into a whole new world on a century timescale? If one could paint a picture for people of the world in a few centuries – wet bulb temperatures beyond human parameters for survival over a majority of the surface, vast deserts, burned forests, inundated cities and farmland, depleted ozone layer, increased tectonic activity, hydrogen sulphide, dead oceans etc – in all probability our numbers reduced to a marginal population clinging onto existence in niche habitats without modern technology… then how important is the car or the central heating or the air conditioning or the foreign holiday?
Moreover it is entirely possible that natural feedbacks we have already committed the earth system to will take us into that future regardless of our actions now.
LikeLike
robertscribbler
/ January 27, 2014There’s a lot in this post, so I’m going to answer it one point at a time over numerous posts.
First, during a hothouse event, the deep ocean warms faster that the surface die to changes in ocean currents. Large pools of much warmer water become sequestered in the depths.
Second, the hydrogen sulphide producing bacteria feed on organic material that rains down continuously from above as creatures in the water column die and their bodies precipitate to the depths. Some anaerobes can also consume methane. But methane is not their only source of food. The deep ocean already contains enough of this material to keep the H2S bacteria going for some time. As noted before, the primary limiter to their expansion is oxygen. And in a warming ocean, oxygen starts to grow scarce which sets off the chain of events leading to an explosion in h2s producing bacteria.
Now with the Arctic, where a high concentration of the methane in question is stored, you already have warmer bottom water conditions. And this feature will intensify as polar amplification continues.
Next up — thoughts on researching concurrent methane h2s release.
LikeLike
ccgwebmaster
/ January 29, 2014Having had more of a look into hydrogen sulphide (though still nowhere near enough), it’s clear you’re nearer the likely ultimate truth in terms of threat magnitude. Definitely looks far more threatening than increased tectonic activity…
I’ll wager there is a shortage of meaningful research into this aspect of things (given even methane is seriously under investigated) but I’d appreciate any and all offhand references to relevant scientific papers from all and sundry… particularly those that might inform extrapolation of the likely rate of onset of such conditions, any geographic variations one might expect, probable atmospheric concentrations, worst case localised releases, and what attributes enabled complex lifeforms to survive the last such event.
LikeLike
robertscribbler
/ January 29, 2014I am making phone calls to the relevant scientific orgs as we speak. In a bit, I should have far more on the way of references than I do now. Preparing a comprehensive post, including sources for the offering.
It is worth noting that it’s tough to get people to talk on the issue. I attribute this to natural fear when faced with a tough issue.
LikeLike
ccgwebmaster
/ February 4, 2014One comment you made was that perhaps people in the future might not be able to risk going down to the sea on account of the potential for toxicity from hydrogen sulphide (which I think remains to be determined somewhat). However – if this article has any pertinence (and I was aware that we can expect almost entirely dead oceans towards the end of the century), there won’t be so much reason to want to be by the sea even in the nearer future – no food…
http://www.cbsnews.com/news/salt-water-fish-extinction-seen-by-2048/
If 1 billion people currently depend on the sea for their protein needs that makes fish stocks as significant in their own way as any of the larger agricultural producer nations.
That raises another question – are you planning to stick with climate change, weather and the arctic as themes in your articles – or will you explore global harvest expectations (and realisations) and the social dynamics at work as things deteriorate? Are you familiar with the NECSI research into the link between food prices and social instability? There was another paper that I do not recall precisely offhand that seemed to go some way to identifying metrics that would categorise nations at risk of falling into that bracket – where food prices combined with adverse economic circumstance result in social breakdown (sometimes accompanied by civil war and consequent additional worsening of the local agricultural situation and larger refugee movements).
I think food (and price thereof) is a significant predictor of problems and will come to define our nearer future, particular when the instability occurs in regions that export large amounts of critical resources on the global markets (oil and phosphate being two such examples). That said, I guess there’s also an outside chance the course of the nearer future is also heavily influenced by by nationalism and resource competition between superpowers (one can see this escalating quietly already).
LikeLike
robertscribbler
/ February 4, 2014I absolutely agree and have written on the issue both here and in Growth Shock.
At this point, the blog is aimed at most recent threats as well as long-term. The long term threats discussed are usually ones not heavily covered in MSM. This is one of the reasons I’m looking more deeply into H2S.
For food, I tend to do an assessment every six months or so. If there’s an imminent threat, then it will come up far more often.
Right now, the major issues are strange weather, sea ice melt, threats to ocean systems, and changes related to rapid polar amplification.
LikeLike
ccgwebmaster
/ February 4, 2014Does your blog permit email follows? I’d be especially interested in not missing the food stuff and the wordpress reader is kinda spammy.
LikeLike
Miep
/ February 4, 2014ccg: go into wordpress.com, click “blogs I follow” and then “edit” and you can have almost any of them emailed to you in various ways, including this one.
LikeLike
ccgwebmaster
/ February 4, 2014Wow, that’s a dreadfully unintuitive interface WordPress has – thanks very much!
LikeLike
Miep
/ February 4, 2014Glad I could help. They have instructions online, but who reads instructions? 🙂
LikeLike
robertscribbler
/ February 4, 2014Thanks, Miep! Was unaware of this wrinkle in the wordpress system.
LikeLike
Miep
/ February 4, 2014You’re welcome, Robert. It took me awhile to stumble across it myself.
LikeLike
PeterC
/ February 4, 2014Thank you for your well-written report.
I’m thinking that the on-going & unstoppable Fukushima radiation discharges into the Pacific Ocean is greatly reducing the Oxygen producing Plankton… less Oxygen, more poison… not good.
There is a website that, for over 700 days now, has been providing a daily report of events related to Methane/Hydrogen Sulphide. It’s straight up. Events reported by media. It is allowing me to monitor the progress of our extinction:
http://jumpingjackflashhypothesis.blogspot.ca/
You may also wish to dig into “Spirit Science” on YouTube… it’s about the only good news I’m aware of.
Peace All.
LikeLike
robertscribbler
/ February 4, 2014It’s an interesting catalog of events. I don’t have enough information to validate these findings but the hypothesis is certainly worth taking a look into. On my side, I’m trying to develop more direct scientific support/observations by known bodies before taking a crack at some of these.
LikeLike
xraymike79
/ February 4, 2014I was wondering how much of the world’s oceans would have to be in euxinic conditions for an extinction event. Apparently not much:
…”Under low-oxygen environments, many biologically important metals and other nutrients are removed from seawater and deposited in the sediments on the seafloor, making them less available for life to flourish.”
“What makes this discovery particularly noteworthy is that we mapped out a landscape of bioessential elements in the ocean that was far more perturbed than we expected, and the impacts on life were big,” said Timothy W. Lyons, a professor of biogeochemistry at UCR, Owens’s former advisor and the principal investigator on the research project.
Study results appear online this week in the Proceedings of the National Academy of Sciences.
Across the event 93.9 million years ago, a major biological extinction in the marine realm has already been documented. Also associated with this event are high levels of carbon dioxide in the atmosphere, which are linked to elevated ocean and atmospheric temperatures. Associated consequences include likely enhanced global rainfall and weathering of the continents, which further shifted the chemistry of the ocean.
“Our work shows that even though only a small portion of the ocean contained toxic and metal-scavenging hydrogen sulfide, it was sufficiently large so that changes to the ocean’s chemistry and biology were likely profound,” Owens said. “What this says is that only portions of the ocean need to contain sulfide to greatly impact biota.”…
http://www.astrobio.net/pressrelease/5789/small-increase-in-hydrogen-sulfide-made-ancient-ocean-toxic-for-life
Any thoughts on this?
LikeLike
robertscribbler
/ February 4, 2014This is very useful. I’ll have to give it a deeper read.
My initial thoughts are that ocean life is very sensitive to these kinds of changes and it doesn’t take much, once the engine gets moving in the wrong direction, to result in a mass die-off in marine environments.
Don’t see too much related to land in this as yet. Will dig deeper. Have a larger report on H2S that I’m working on so thanks for the addition.
LikeLike
iconickevin
/ May 3, 2014I’m incredibly underqualified to comment on this article and the epic debate ensuing but I feel very privilidged to be able to follow it. I have completed 16 Ocean passages on Yachts in the Pacific and feel very attached/ grounded in it. I have witnessed sub marine Volcanic eruptions in the Tongan trench and swum in heated Sea water adjacent to the Volcano in Tanna which one day swallowed a crayfish I was steaming in a rock pool on the coast!!!
We considered it a donation to the Volcano spirit and as we stood there it ejected a few Crayfish/lobster legs cooked and ready to eat!
Awesome debate everyone, Thx.
LikeLike
Robert Schmidt
/ June 22, 2015Is there any chance of ocean engineering to stall or lessen the H2S nightmare?
I know at one point there was talk of fertilizing ocean waters with iron to encourage CO2 sequestration.
Would introducing O2 at ocean depths lessen or eliminate the H2S nightmare?
LikeLike
robertscribbler
/ June 22, 2015Fertilizing the ocean with iron results in algae blooms which results in ocean anoxia, which speeds the transition to a hydrogen sulfide producing state. And the kinds of volume you would need to sequester enough cabin to matter would really speed the progression toward a Canfield type ocean. Introducing oxygen to the deep ocean would only help if the deep ocean was cool enough to hold the oxygen in solution. In addition, the volume of oxygen needed would be extraordinary. Where would you get it? As with most gel-engineering schemes — better and easier just to stop burning fossil fuels.
LikeLike
Exposing the Big Game
/ January 3, 2016Reblogged this on Exposing the Big Game.
LikeLike
Liz
/ March 18, 2016Yesterday in Southern California, people reported rotten egg gas smell coming from the ocean.
http://www.latimes.com/socal/daily-pilot/news/tn-dpt-me-0318-gas-leak-20160317-story.html
Hydrogen sulfide?
LikeLike
Simon
/ April 8, 2017Your blog is the first I check every day. This post is a reminder why.
LikeLike
Sunwyn Ravenwood
/ February 15, 2018It is spelled Cthulhu. Other than that, great article. “Under a Green Sky” was the scariest book I ever read.
LikeLike